Hybridization in biophotonics and imaging: revealing the ultrastructures and understanding the functions
Coordinator : Pierre NASSOY (LP2N)
credit to: R. Fronzes, P. Bon and K. Alessandri
Superresolution fluorescence microscopy and cryogenic electron are powerful imaging methods for exploring the subcellular organization of biomolecules Superresolution fluorescence microscopy based on labeling highlights specific proteins and has sufficient sensitivity to observe single fluorescent molecules, but the reconstructions lack detailed cellular context.
Cryo-electron microscopy has molecular-scale resolution but lacks specific and non-perturbative intracellular labeling techniques. The combination of these two techniques, in their 3D-resolved configurations, is therefore a powerful approach for observing the subcellular arrangement of biomolecules.
Platforms that hybridize state-of-the-art fluorescence nanoscopies with label-free imaging approaches (e.g. OC), quantitative phase), or with specific methods for applying and/or measuring environmental stress will allow to perform quantitative functional imaging and to extract specific biophysical parameters in relevant physiological conditions.
These technological developments require prior refinement studies performed on bioengineered model samples (e.g. organoids, active substrates) and on simple biological systems (e. g. cell adhesion sites in mechanotransduction, transport complexes in bacteria) prior to their use to address issues in the fields of neurobiology, oncology and for the study of pathogens.
This major research theme is organized into three main axes.
1. Hybridization between complementary modalities bearing inherent constraints
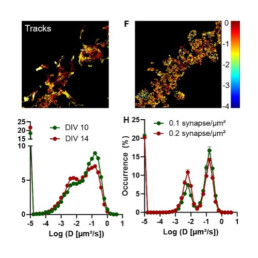
One goal of ultrastructural imaging by cryo-electron microscopy (CEM) lies in the identification of molecules of interest within the highly resolved but hardly specific images that this technique is able to provide. We use fluorescence labeling and super-resolution imaging to localize the positions of these molecules within the cryo-electron microscopy images. This requires working at ultralow temperature during the whole imaging process. The Bordeaux campus has world-renowned expertise in low-temperature imaging of individual molecules. We develop 3D cryogenic nanoscopy of cryofixed biological samples to image proteins with high 3D precision and perform correlative cryo super-resolution light and electron microscopy. This work package is also supported by the NanoCryoCLEM EquipEx+.
The second aim is to perform functional imaging at physiological temperature. This requires to minimally disturb the sample during the measurement. This implies to keep light excitation to a minimum and to use stealth imaging probes. We combine label-free approaches with super-resolved imaging techniques based on the use of innovative stealth and photo-controlled single nanoparticle probes.
2. The challenge of quantification
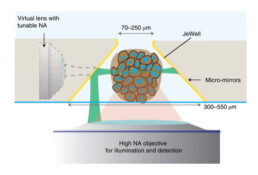
Imaging of a sample should not be limited to qualitative observations. It must be accompanied with a robust and meaningful workflow to extract quantitative information relevant to biological questions. The challenge for Label-free optical techniques is to go beyond imaging and to extract from the images physical parameters that have biological significance. These techniques need to be able to reveal a specificity (molecular, chemical or physical) free from the inevitable constraints associated with each modality which may result from sample preparation or from the instrument itself. Since biological samples are by nature three-dimensional objects, the co-development of imaging techniques capable of delivering 3D resolved images while working at depth is therefore an important development of this work package.
3. Model and Flagship biological applications
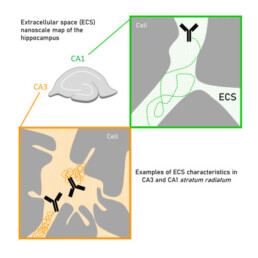
The instrumental approaches developed in the previous work packages are validated on biological objects that are well-controlled or extensively investigated by local biologists? These include for instance ii) focal adhesion sites between cells and extracellular matrix that exhibit a specific nanometric organization, ii) tumor spheroids or stem-cell derived organoids that have a radial cell assembly dependent on nutrient uptake, drug treatment and growth, iii) the activity and redox properties of mitochondria as subcellular structures.
Teams
IBGC
Bordeaux Imaging Center
Selected Publications
Automated high-speed 3D imaging of organoid cultures with multi-scale phenotypic quantification
Beghin A, Grenci G, Sahni G, Guo S, Rajendiran H, Delaire T, Mohamad Raffi SB, Blanc D, de Mets R, Ong HT, Galindo X, Monet A, Acharya V, Racine V, Levet F, Galland R, Sibarita JB, Viasnoff V. Nat Methods. 2022 Jul;19(7):881-892. doi: 10.1038/s41592-022-01508-0.